Research focus
The MycoSym Lab- Symbiotic plant physiology
Plants are immobile organisms that rely on exceptional developmental and physiological plasticity to survive in variable and often unpredictable environments. This plasticity arises from the coordinated regulation of hormonal signalling, transcriptional networks, metabolism, and development, which enables plants to integrate external biotic and abiotic cues within adaptive growth programs. At the MycoSym Lab, we study the fundamental mechanisms by which plants translate biotic cues into physiological responses that support growth, nutrient use efficiency, and resilience at both organismal and ecosystem scales.

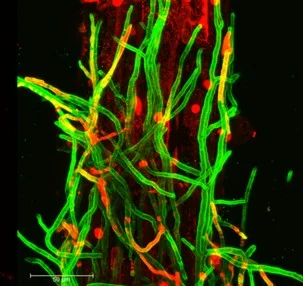
A central research direction in the lab focuses on beneficial symbiotic fungi as key regulators of plant physiological acclimation. Using an evolutionarily early-diverging soil fungus that associates with a broad range of plant hosts, we examine how fungal-derived signals reshape plant hormone networks, nutrient uptake pathways, and root system architecture. This system provides a powerful framework to explore conserved strategies by which microbial partners enhance plant performance and to understand how symbiosis contributes to acclimation across diverse environmental contexts and evolutionary lineages.
A complementary approach focuses on arbuscular mycorrhizal fungi, which form one of the most widespread and ecologically important symbioses in the plant kingdom. Here, we study how fungal effector proteins actively modulate host cellular and physiological processes during symbiosis. By identifying and functionally characterizing fungal effector proteins, we examine how symbiotic partners actively reprogram host hormone signalling, carbon allocation, and nutrient transport. We aim to reveal molecular strategies that underpin efficient nutrient exchange and highlight how symbiotic regulation selectively reprograms to support efficient nutrient exchange and contribute to ecosystem functioning and crop performance.


Across these systems, our laboratory integrates molecular biology. genetics, cell biology, advanced imaging, and high-throughput phenotyping. By examining how symbiotic signals influence nutrient uptake, carbon allocation, root system architecture, and growth dynamics, we link cellular and molecular mechanisms to whole-plant performance under varying environmental conditions. This physiological framework allows us to understand how plant-microbe interactions shape nutrient-use efficiency, stress tolerance, and productivity, providing insight into plant function.
Lab members
Mamoona Khan PhD (Principle investigator)
Yun Tang (PhD student)
Ibrahim Bakr (Masters Student)
Kaviya Thanikathan Subramanian (Student helper)
Past members
Nesrin Kaiser (Masters Student, 2025)
Matthia Comparini (Student helper, 2025)
Selected publications
For a full list of publication please visit:
Orcid: https://orcid.org/0000-0003-3301-9536
Google Scholar: https://scholar.google.com/citations?user=d5uhCusAAAAJ&hl=en
*Equal contribution, # corresponding author
-
Khan M#, Nagarajan N, Schneewolf K, Marcon C, Wang D, Hochholdinger F, Yu P, Djamei A#. Pathogenic fungus Ustilago maydis exploits the lateral root regulators to induce pluripotency in maize shoots. 2025. New Phytologist (2025) Dec 26. https://doi.org/10.1111/nph.70843 . Online ahead of print.
-
Khan M#. Gatekeeping symbiosis: autophagy shapes Serendipita indica -Arabidopsis thaliana interaction. Plant Physiology. 2025 Oct 31;199(3):kiaf593. https://doi.org/10.1093/plphys/kiaf593.
-
Khan M#. Belowground podcast: Digital droplet PCR to estimate root biomass and species profiling from soil samples. Plant Physiology. 2025 Jul 3;198(3):kiaf313. https://doi.org/10.1093/plphys/kiaf313
-
Khan M#. N matters: Insights into nitrogen assimilation from the ectomycorrhizal fungus Laccaria bicolor. Plant Physiol. (2025) May 30;198(2):kiaf231. https://doi.org/10.1093/plphys/kiaf231
- Khan M, Uhse S, Bindics J, Kogelmann B, Nagarajan N, Tabassum R, Ingole KD, Djamei A. Tip of the iceberg? Three novel TOPLESS-interacting effectors of the gall-inducing fungus Ustilago maydis. New Phytologist (2024) Nov;244(3):949-961. https://doi.org/10.1111/nph.19967
- Khan M, Djamei A. TOPLESS Corepressors as an Emerging Hub of Plant Pathogen Effectors. Mol Plant Microbe Interact. (2024) Mar;37(3):190-195. https://doi.org/10.1094/MPMI-10-23-0158-FI
- Khan M#., Djamei A. Co-immunoprecipitation based identification of effector-host protein interactions from pathogen-infected plant tissue. Methods Mol Biol. 2023;2690:87-100. (2023). Springer. https://doi.org/10.1007/978-1-0716-3327-4_8
- Khan M#., Djamei A. Performing Infection Assays of Sporisorium reilianum f. sp. Zea in Maize. Methods Mol Biology;2494:291-298 (2022). https://doi.org/10.1007/978-1-0716-2297-1_20
- Bindics J*., Khan M*., Uhse S., Kogelmann B., Baggely L., Reumann D., Ingole K., Stirnberg A., Rybecky A., Darino M., Navarrete F., Doehlemann G., Djamei A. Many ways to TOPLESS – manipulation of plant auxin signalling by a cluster of fungal effectors (2022). New Phytologist. https://doi.org/10.1111/nph.18315
- Khan, M, Rozhon, W, Unterholzner, SJ, Chen, T, Eremina, M, Wurzinger, B, Bachmair, A, Teige, M, Sieberer, T, Isono, E and Poppenberger, B. “Interplay between phosphorylation and SUMOylation events determines CESTA protein fate in brassinosteroid signaling”. Nature Communications (2014) Aug 19;5:4687. DOI: 10.1038/ncomms5687
- Khan, M, Rozhon, W, and Poppenberger, B. Mini-Review ‘The role of hormones in the aging of plants. Gerontology 2014 ;60(1):49-55. DOI: 10.1159/000354334
- Khan, M, Rozhon, W, Bigeard, J, Pflieger, D, Husar, S, Pitzschke, A, Teige, M, Jonak, C, Hirt, H and Poppenberger B. “Brassinosteroid-regulated GSK3/shaggy-like kinases phosphorylate MAP kinase kinases, which control stomata development in Arabidopsis thaliana”. Journal of Biological Chemistry (2013) Mar 15;288(11):7519-27. DOI: 10.1074/jbc.M112.384453
This publication was selected by the "Journal of Biological Chemistry" (JBC) as one of the 22 best papers of 2013 from more than 4000 JBC publications https://www.jbc.org/best-of-2013
- Poppenberger B*, Rozhon W*, Khan M*, Husar S, Adam G, Luschnig C, Fujioka S and Sieberer T. CESTA, a positive regulator of brassinosteroid biosynthesis. EMBO Journal 2011 Mar 16;30(6):1149-61. DOI: 10.1038/emboj.2011.35